Roald Dahl’s letter about his 7-yr-old dying from measles is a must read for our time
The children’s book author doesn’t mince words.
Roald Dahl's daughter contracted measles the year before the vaccine was invented.
On February 26, 2025, officials announced that a child in Texas had died of measles, becoming the first death from the disease in the U.S. in a decade. A local outbreak among unvaccinated people highlights the dangers of anti-vaccine sentiment that has affected vaccine rates and opened the door to a highly infectious disease that was considered eliminated in the U.S. in 2000.
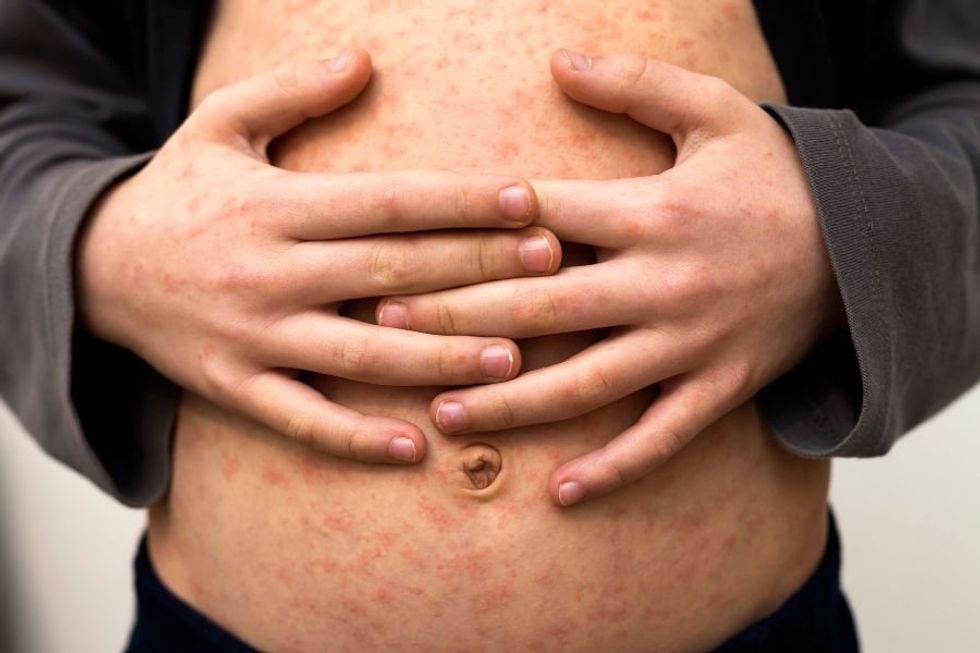
A century ago, before the measles vaccine was developed and distributed, measles was a common childhood disease that nearly everyone caught at some point. But it wasn't harmless. Of the 3 to 4 million cases per year, 48,000 people were hospitalized and 400 to 500 people died. According to Johns Hopkins Bloomberg School of Public Health, the disease itself is dangerous and can also lead to pneumonia, encephalitis, brain damage, and other health problems.
Children's author Roald Dahl lost his 7-year-old daughter Olivia to measles-induced encephalitis in 1962, the year before the measles vaccine was invented. Over two decades later, he wrote a heart wrenching letter about her death, encouraging people to vaccinate their children to avoid that now-preventable tragedy.

He wrote:
"My eldest daughter caught measles when she was seven years old. As the illness took its usual course I can remember reading to her often in bed and not feeling particularly alarmed about it. Then one morning, when she was well on the road to recovery, I was sitting on her bed showing her how to fashion little animals out of coloured pipe cleaners, and when it came to her turn to make one herself, I noticed that her fingers and her mind were not working together and she couldn’t do anything.
'Are you feeling all right?' I asked her. 'I feel all sleepy,' she said.
In an hour, she was unconscious. In 12 hours she was dead.
The measles had turned into a terrible thing called measles encephalitis and there was nothing the doctors could do to save her. That was 24 years ago in 1962, but even now, if a child with measles happens to develop the same deadly reaction from measles as Olivia did, there would still be nothing the doctors could do to help her.
On the other hand, there is today something that parents can do to make sure that this sort of tragedy does not happen to a child of theirs. They can insist that their child is immunised against measles. I was unable to do that for Olivia in 1962 because in those days a reliable measles vaccine had not been discovered. Today a good and safe vaccine is available to every family and all you have to do is to ask your doctor to administer it.
It is not yet generally accepted that measles can be a dangerous illness.
Believe me, it is. In my opinion, parents who now refuse to have their children immunised are putting the lives of those children at risk…It really is almost a crime to allow your child to go unimmunised."

Dahl wrote his letter to parents in the United Kingdom in 1988, but it's just as relevant today. While some parents worry about the side effects of vaccines and rumors about the MMR (Measles, Mumps, and Rubella) vaccine being linked to autism, studies have shown that there is no evidence that the MMR vaccine causes autism and according to Johns Hopkins, "Measles is a dangerous disease and the vaccine is very safe. The risks of severe illness, death, or lifelong complications from measles infection far outweigh the generally mild side effects some people experience following vaccination. Serious reactions to the MMR vaccine are rare." There is still no cure for measles or measles encephalitis.
Dahl dedicated two of his books to Olivia: James and the Giant Peach when she was still alive and The BFG after her death. Though his books have long been controversial and his legacy has been marred by antisemitism and racism that his family felt the need to formally apologize for, Dahl was right about vaccines. His experience losing his daughter serves as a cautionary tale for those who may be tempted to take the drastic reduction in infectious diseases due to vaccines for granted.